Metal induced energy transfer with wide-field microscopy
Introduction
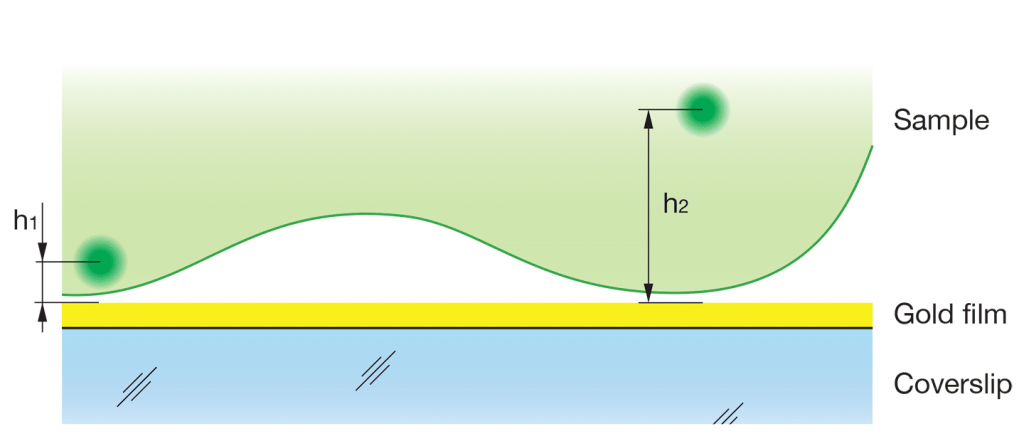
In Förster resonance energy transfer (FRET) an excited donor molecule transfers energy to an acceptor molecule. In MIET technique a metal plasmon surface acts like an acceptor allowing to measure distances with nanometer accuracy 1. On the figure a general MIET setup is depicted. A gold film coating of a coverslip acts as an energy acceptor by efficiently quench- ing the fluorescence of chromophores of the sample. The efficiency of the energy transfer depends on the distance between the molecule and the surface: the closer the chromophore is located to the gold layer, the higher the probability of the transfer. This efficiency is observable as a change of the lifetime of the excited state allowing one to measure the distances h1 and h2. The reported efficient depth range of this technique is up to 100 nm matching to the typical working light penetration depth in total internal reflection fluorescence (TIRF) microscopy.
Fixed cell measurement
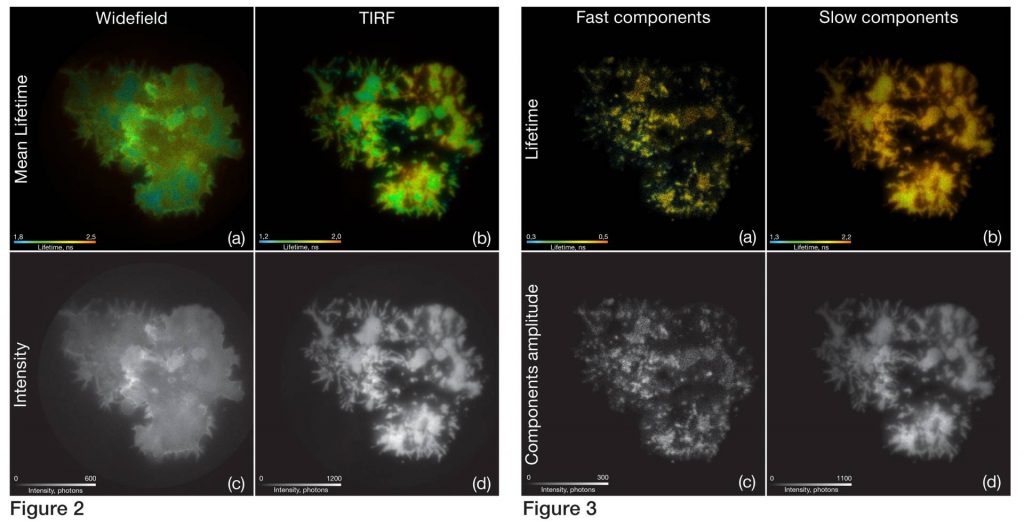
T-lymphocytes transfected with a GFP marked protein kinase (GFP-Lck) were fixed on gold coated coverslips. First, measurements were made under standard epifluorescence illumination conditions with 488 nm excitation wavelength. The resulting intensity weighted mean lifetime (Fig. 2a) and the intensity (Fig. 2c) images show the typical lifetime and grey value range for GFP with a slight quenching on the contact sites. The second measurement of the same cells was performed with TIRF illumination revealing much higher lifetime contrast (Fig. 2b) related to MIET effect.
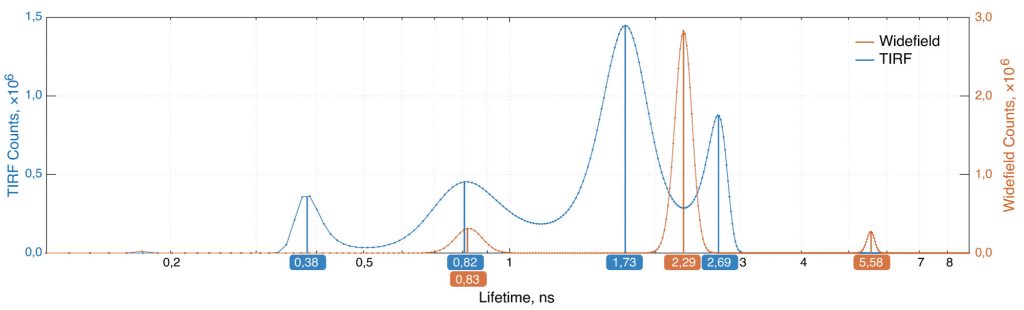
To discriminate fluorescence lifetime components, we performed a maximum entropy method (MEM) analysis (Fig. 4). The results of per pixel amplitude fitting on basis of the MEM analysis are shown in figure 3.
The amplitudes corresponding to the lifetime components of 0,38 ns and 0,82 ns were summed into the intensity image (Fig. 3c) and the amplitude weighted lifetime map (Fig. 3a). The results for two other components of 2,29 ns and 5,58 ns are shown as well (Fig. 3b, 3d). The short components images demonstrate typical clustering of the protein kinase along the plasma membrane after T-cell receptor stimulation.
Publications
[1] A. Chizhik, J. Rother, I. Gregor, A. Janshoff and J. Enderlein, Metal-induced energy transfer for live cell nanoscopy, Nat. Photonics, Vol. 8, 2014
DOI: 10.1038/nphoton.2013.345
